Healy Device: A Class 2a Medical Device in Europe and Its Global Journey
The Genesis of Healy: Conceived as a Medical Device
From its inception, the Healy Device was destined to be more than just another gadget; it was engineered to meet rigorous medical standards. Developed by German engineers specializing in medical devices, the journey began with meticulous planning and design. The entire development process was overseen by companies certified in medical device development, manufacturing, and assembly—all based in Germany.
This video describes in detail how the Healy Device became a class 2a medical device in Europe and its approval process as a wellness device in other countries around the world.
Certification: The Rigorous Path to Approval
Achieving Class 2a medical device status in Europe is no small feat. It involves a series of stringent tests and audits, conducted annually by notified bodies, to ensure compliance with the highest standards. For Healy, this included extensive electrical and electromagnetic safety tests, aligning with international standards for medical device safety.
Healy’s Clinical Validation
Before receiving approval, Healy was subjected to a comprehensive clinical evaluation as per the latest European guidelines. This evaluation included nearly 60 studies, demonstrating the device's efficacy in treating conditions like chronic pain, fibromyalgia, skeletal pain, migraine, and supporting mental health treatments for conditions such as depression and anxiety. The evaluation not only verified its effectiveness but also ensured its safety for public use.
Global Approvals and Varied Applications
Following its success in Europe, Healy gained approval in countries across the globe including the USA, Canada, New Zealand, and several others. Each country's approval process scrutinized Healy’s technical documentation and clinical evaluations afresh, adapting to local regulations which influenced the indications for which it could be approved.
A Wellness Device Around the World
In countries where Healy is not approved as a medical device, it takes on a different role as a wellness device. Known as "Healy Wellness," it offers programs to promote well-being, allowing users to benefit from the technology even without medical certifications.
Ongoing Research and Development
Healy World continues to advance its research, conducting post-market clinical follow-up studies to ensure continued efficacy and safety. These studies are crucial for maintaining certification and for evolving product efficacy. Although not all studies meet the stringent requirements for medical device approval, they still provide significant evidence of Healy's potential benefits in enhancing vitality and overall well-being.
The Future of Healy: Continuous Innovation and Global Expansion
The journey of the Healy Device is far from over. With ongoing studies and a commitment to high standards, Healy World aims to ensure that each of its products contributes positively to the physical, mental, and emotional wellness of its users. As Healy continues to evolve, it aims to not only meet existing standards but also to redefine what is possible in the convergence of health technology and holistic wellness approaches.
Healy: A Model of Medical and Wellness Innovation
The story of the Healy Device serves as a compelling example of how rigorous scientific processes, combined with innovative technology, can lead to significant advancements in both medical and wellness fields. As Healy continues to be recognized globally, it stands as a testament to the potential for technology to transform lives, demonstrating a successful balance between strict regulatory compliance and the pursuit of holistic health and wellness.
¡Noviembre Explosivo! Healy World México trae el Black Friday 2025 con descuentos de hasta el 50% en ediciones como Healy Resonance Plus y Professional, además de regalos exclusivos como el Healy Coil y el Módulo Meridianos. Descubre los Combos Duo y Élite, y paga hasta en 24 Meses Sin Intereses. ¡Válido del 27 al 30 de Noviembre!
Discover the exclusive Healy Consciousness Bundle featuring four powerful Digital Modules: Essential Oils , Personal Growth , Plant Power , and the Animal Module. Available for a limited time in the USA and Canada.
The Healy Professional Edition is 50% OFF for a limited time! Get the most comprehensive model for only SG$ 2,686.50. Shop the ASIA Black Friday sale before Nov 30.
The annual Healy Black Friday Sale is here! Save 15% on Healy Discover, Evolve, and Pro Editions and get 20% off Ultimate and Elevate Bundles for customers in the UK, Europe, and UAE. Use the exclusive promo codes before November 30th.
Announcing the worldwide launch of MagHealy Obsidian! We're offering a limited-time Founder Bundle: buy two devices and save 35%. This new edition features 110 frequency programs, lifetime access to updates, and an exclusive VIP club membership.
¡Healy Colombia Shop celebra su primer año con una promoción de aniversario sin precedentes! Obtén un 60% de descuento en Healy Professional y MagHealy Professional. Además, añade un Healy Coil con 50% de descuento. Descubre este Healy Discount especial y únete a la celebración del bienestar en Colombia. Válido durante todo agosto
This July, unlock unparalleled wellness with Healy Obsidian! Explore fantastic promotions worldwide, including bundled Healy Device offers with MagHealy Professional, Meridian Modules, and exclusive benefits in North America and Asia.
Healy Americas customers, get ready! Our incredible Flash Summer Sale offers a 60% discount on the Healy Professional Device. This is your chance to purchase the Healy Professional at its lowest price ever! Available for a limited time in the USA, Canada, Mexico, and Colombia. See discounted prices for each country inside!
Unsure about the Healy HighWave? We answer your top questions about this revolutionary wireless wearable, designed to enhance your Healy frequency experience and support full-body wellness.
Healy Shop USA Promotion: Get up to 24 months interest-free on Healy Device purchases during our exclusive Klarna promotion! Available only for US residents from June 13th to 23rd, 2025.
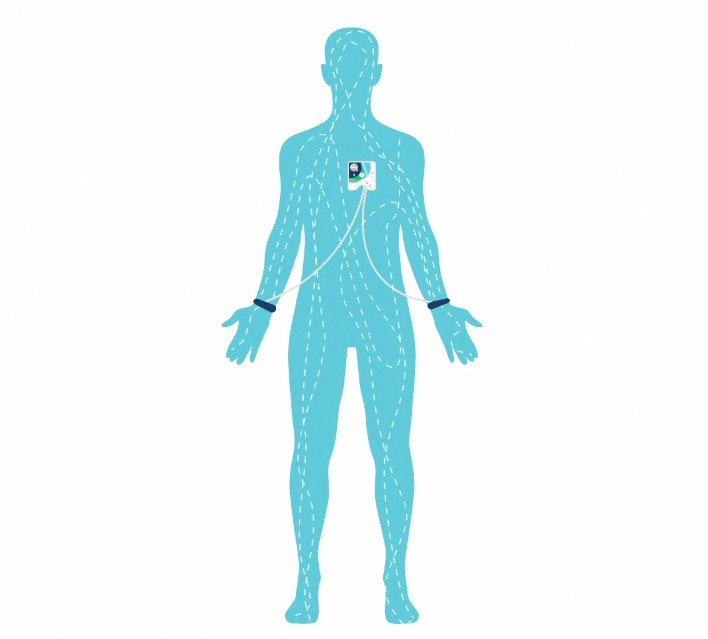
Unleash the full potential of your Healy device with the new Healy HighWave wearable. Learn how this innovative accessory delivers frequencies from head to toe, featuring exclusive Mind Wave and Cell Wave programs.
Want to experience Healy's innovative frequencies but prefer to manage your payments? Now you can! For a limited time, US residents can purchase any Healy device using Klarna and benefit from 0% interest for up to 24 months. Get started on your wellness journey without the upfront cost. Offer valid until April 30th, 2025. Don't forget to explore our additional discounts in the Healy USA Shop!
Limited-time Healy promotions in Mexico! Get up to 50% off Healy Professional and deals on Resonance Plus & Resonance this April. Exclusive VIP Club offer for Obsidian buyers. Visit our Healy Mexico shop!
Huge savings on Healy Devices in Canada this April! Don't miss our Perfect Pair & Resonance bundles with free gifts. Limited time offer!
Healy World is rapidly expanding globally! Check our updated list of open markets in the Americas, Europe, and Asia Pacific to see if you can purchase a Healy device in your country. Find your official local shop now.
Exclusive Healy India sale! Don't miss incredible discounts on Healy Professional, Resonance, and valuable free accessories. Visit Healy Shop India!
Healy World India kicks off 2025 with amazing discounts! Grab a FREE MagHealy Professional with the Obsidian, save 40% on bundles, and get 30% off select Healy Editions. Plus, free coils and more! Limited-time offers end January 31st.
Discover Healy World's innovative supplement strips, Elements, as featured in L'Officiel England. Learn how these strips work with Healy's frequency technology to optimize your well-being.
Healy Colombia: New Year, New You! This January, experience the power of Healy frequency devices with incredible discounts! Save up to 45% on select editions and receive exclusive free gifts. Shop now and start your journey to a healthier, happier you!
Discover the transformative power of Solfeggio frequencies! Learn how the Solfeggio Frequency Music Player retunes your favorite songs into healing frequencies like 528 Hz and 432 Hz for relaxation, better sleep, and wellness.
Don't miss out! Incredible Healy India promotions end soon! Grab a FREE MagHealy Pro or Healy Resonance Plus with select purchases. Plus, get up to 40% OFF Healy devices and bundles. Shop now before these deals disappear!
Celebrate the end of year with amazing Healy World promotions in Australia! Grab a Healy Device for yourself or a loved one with generous discounts and free gifts. Healy Obsidian Edition, Resonance, and more included in our Healy Sale!
The Healy Business Suite Module is your ultimate tool for personal growth and business success. With cutting-edge Information Field technology and four specialized modules, it empowers you to overcome challenges, optimize goals, and thrive at every stage of your career.
Exciting news! Healy, the revolutionary wearable wellness device, is now officially available in Cambodia. As of December 10th, 2024, Healy World Cambodia is open for business!
Get your Healy Device in the UAE! Shop via the Healy Europe store with direct shipping and access exclusive Healy discounts. Find prices for all editions, including Healy Obsidian, Healy Resonance, and Gold, along with the MagHealy Device.
Black Friday is here, and Healy India is celebrating with incredible deals! Get a FREE MagHealy Pro with Healy Obsidian, 50% off MagHealy Professional Bundles, 30% off Healy Editions, and more. Shop now!




























